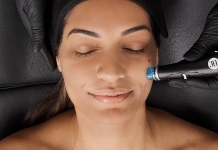
FDA approval for SkinPen microneedling
 The SkinPen® Precision Microneedling Device has gained FDA approval, setting a new standard as the only recognised Class II microneedling device available.
The SkinPen® Precision Microneedling Device has gained FDA approval, setting a new standard as the only recognised Class II microneedling device available.
According to manufacturer Bellus Medical, the SkinPen® is the first and only microneedling device to receive this important designation from the U.S. Food and Drug Administration (FDA).
Joe Proctor, Bellus Medical President and CEO says:
"FDA clearance for SkinPen Precision demonstrates our unwavering commitment to the safety, quality and excellence needed to elevate the standards in the microneedling industry. With this recognition by the highest organisation in the U.S., the FDA, healthcare providers and consumers know they can trust SkinPen Precision and Bellus Medical to create, develop and manufacture the safest and most cutting-edge solutions on the market.”
Microneedling, which is sometimes also referred to as collagen induction therapy, offers a minimally invasive skin rejuvenation procedure that helps minimise signs of ageing, whilst improving the appearance of acne scars and rejuvenating skin. The device works by causing controlled micro-injuries to the skin which induce the body's natural healing process. This process triggers the release of cytokines and growth factors that lead to remodelling of collagen and elastin.
The short procedure is suitable for all skin types and tones, and requires little downtime.
In order to achieve FDA approval, Bellus Medical worked through a rigorous three-year evaluation process meeting more than 90 validation requirements for the microneedling device, charger base and proprietary cartridge.
Jennifer Block, Bellus Medical Director of Quality & Regulatory, adds:
"Our evaluation process with the FDA resulted in a new draft guidance issued by the FDA, which includes regulatory requirements for microneedling devices. Our steadfast focus on healthcare provider and patient safety resulted in a new classification for microneedling devices. Bellus Medical's SkinPen Precision is truly leading the industry as the only microneedling system on the market meeting the FDA's new standards."
The SkinPen® Precision Microneedling Device is available exclusively in the UK from BioActiveAesthetics.

-13546.png)