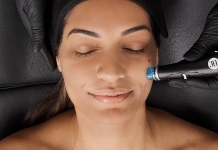
Microneedling device gains efficacy certification

Microneedling system, SkinPen® Precision, has gained the British Standards Institution (BSI) Class IIa CE Certification mark, confirming the
SkinPen® as a treatment to improve the appearance of facial acne scars in adults aged 22 years or older, and to improve the appearance of fine lines and wrinkles on the face and neck.
The
SkinPen® is also intended to treat pigmentation conditions (dyschromia) including Melasma, Vitiligo and Solar Lentigines.
The device is used in over 950 clinics across the UK clinics, and has been developed by Crown Aesthetics, a division of Crown Laboratories, Inc. The company runs robust clinical trial programs to scientifically validate new product indications.
Commenting on achieving Class IIa CE Certification, Joe Proctor, President of Crown Aesthetics, says:
“This new and expanded certification further validates SkinPen's safety and usage claims in the EU. With SkinPen's advanced microneedling technology, we continue to set a higher standard in patient care, and firmly establishes the industry benchmark for safety. This essential certification allows us to continue offering best in class technology to our physician partners and their patients and solidifies the company's growing presence in the global market.”
SkinPen® Precision is distributed in the UK by BioActiveAesthetics.

-13546.png)